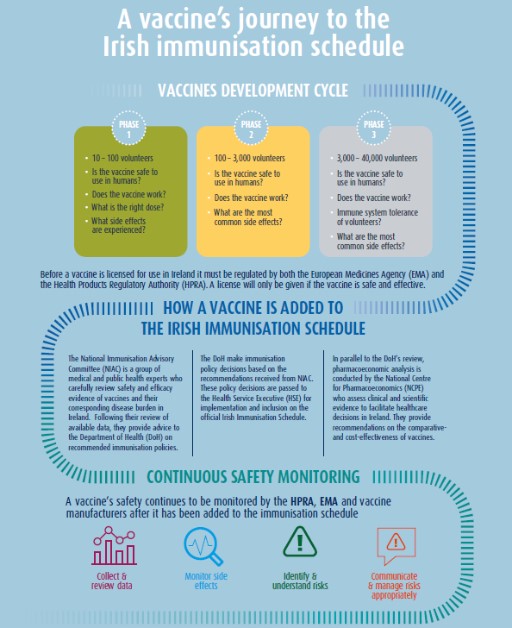
All vaccines in Ireland are licensed by the European Medicines Agency(EMA) and the Health Products Regulatory Authority (HPRA).
The European Medicines Agency (EMA) was established in 1995 and is an EU agency currently based in London. The Agency is responsible for thescientific evaluation, supervision and safety monitoring of medicinesincluding vaccines developed by pharmaceutical companies for use in the EU.
The HPRA (formerly the Irish Medicines Board) was established in 1996 and is the Irish body responsible for ensuring that all medicines and health products licensed for use in Ireland are as safe as possible and dowhat they are intended to do.
Under its remit to approve and regulate the licensing of human medicines the HPRA is responsible for ensuring the safety of human vaccines.
Coupled with the HPRA and the EMA, the World Health Organisation’s Global Advisory Committee for Vaccine Safety (GACVS), which was established in 1999, has a role in overseeing the global safety of allvaccines.
Before a vaccine is licensed for use in Ireland it must be regulated by both the HPRA and the EMA. Once vaccines are licensed both agencies and the vaccine manufacturers continue to monitor and supervise their safety.
